FDA looks into dental device after KHN-CBS News investigation into patient harm

Following a joint investigation by KHN and CBS News into a dental appliance that numerous lawsuits allege caused serious harm to patients, the FDA began looking into the product, the Anterior Growth Guidance Appliance, or AGGA, according to a former agency official. .
Additionally, KHN and CBS News learned that the Las Vegas Institute, a training company that previously trained dentists in the use of the AGGA, is now training dentists in the use of a different device, which it described as “almost exactly the same device.” This is called Anterior Remodeling Appliance, or ARA.
The FDA’s interest in AGGA was reported by Kara Tenenbaum, a former senior policy adviser at the agency’s device center, who said the FDA should investigate the product, which, according to court records, has been installed in more than 10,000 dental patients.
Tenenbaum said she was contacted by “very concerned” FDA officials after KHN and CBS News released their report, who said they had begun “looking into” AGGA but had not yet determined what legal authority the agency had to regulate it. .
“The FDA is looking into what powers they can have over this device — what they can do,” Tenenbaum said. “Now, of course, whether this device is FDA regulated or not, it still has to be safe.”
KHN and CBS News reviewed online reports confirming that an FDA official spoke with Tenenbaum about AGGA. The FDA declined to comment on the AGGA or confirm whether it evaluates the device.
The AGGA investigation by KHN-CBS News included interviews with 11 patients who said they were harmed by the device, lawyers who said they represent or represented at least 23 others, and dentists who said they examined patients who experienced severe complications while using AGGA. The investigation also found no records of AGGA registration with the FDA, despite the agency’s role in the regulation of medical and dental devices.
AGGA’s inventor, Tennessee dentist Dr. Steve Galella, testified in court under oath that the device was never submitted to the FDA, which he believes has no jurisdiction over it. Tenenbaum said the lack of registration is “incredibly problematic” because it’s one of the methods by which the FDA gathers reports of negative effects from the device.
She urged anyone who witnessed complications from AGGA to help the FDA by submitting a report through the MedWatch portal.
“Whether it’s a dentist, orthodontist, surgeon, patient, family member or caregiver,” Tenenbaum said, “anyone can and should submit these reports so the FDA has a better understanding of what’s going on.”
Victor Krauthamer, a former FDA official who worked for the agency in the medical device regulatory department for three decades, said an FDA investigation into a device typically begins by examining the limits of the agency’s authority, ensuring that any future action is legally enforceable. solid legal base.
Krauthamer said he expects the FDA to take regulatory action against AGGA, including sending a warning letter to the manufacturer and possibly taking control of the devices.
“At this point, I would be surprised if there weren’t any compliance actions, such as a seizure,” Krauthamer said, adding later, “I think the FDA is probably trying to create a case that will keep in judgment and will not be thrown out.”
Jeffrey Oberlis, an attorney for manufacturer AGGA Johns Dental Laboratories, said in an email that the company “looks forward to resolving the charges against it” and declined to comment on AGGA’s or the FDA’s interest in the device.
Galella said in his deposition that he personally used the AGGA on over 600 patients and taught other dentists how to use the device for many years. In videos of one workout during the AGGA trial, Galella said the device puts pressure on a patient’s palate and causes an adult’s jaw to “realign” forward, making them more attractive and “curing” common ailments such as sleep apnea. and temporomandibular joint disorder, or TMJ.
“It’s okay to make a ton of money,” Galella told the dentists in the video. “You are not ripping anyone off. You heal them. You help them. You make their lives beautiful forever and ever.”
Dentists across the country rely on Galella’s teachings on their websites, often claiming that AGGA can “grow”, “reconstruct” or “expand” an adult’s jaw without surgery. At least 11 of these dentists’ websites have apparently removed any mention of AGGA since the KHN-CBS News investigation was published on March 1.
“I think that’s good to hear,” said Boya Kragulj, a former professional clarinetist who claimed in a lawsuit that AGGA caused catastrophic damage to her teeth. “I think when you take this information from patients who are looking for a device and see these claims, it’s generally a good thing.”

Cragul is one of at least 20 AGGA patients who have filed lawsuits against Galella and other defendants over the past three years, alleging that AGGA does not and cannot work. The plaintiffs allege that instead of expanding their jawbones, AGGA left them with damaged gums, loose teeth, and shattered bone.
Some claim in lawsuits that they will lose teeth to the device and add in interviews that they no longer have enough bone to replace those teeth with dental implants.
“Now I can take my finger and literally move my front teeth,” said Melanie Pappalardi, 28, who said she wore AGGA for a year and filed a lawsuit in Indiana. “I can’t bite off absolutely nothing.”
In their lawsuits, the plaintiffs do not allege that Galella treated them, but that he or his company consulted with each of their dentists about their AGGA treatment.
Galella declined to be interviewed by KHN and CBS News. His attorney, Alan Fumuzo, said in a written statement that AGGA is “safe and can give positive results.”
All AGGA lawsuits are ongoing. Lawyers for Galella and his company Facial Beauty Institute deny liability in the lawsuits. Johns Dental Laboratories settled one claim for an undisclosed amount but continues to fight charges in other cases. The Las Vegas Institute, which previously ran AGGA courses for dentists and promoted the device on Facebook, has denied liability in court and is pending a motion to dismiss claims in one lawsuit in which it is named as a defendant.
In an affidavit filed as part of that lawsuit, Las Vegas Institute CEO Dr. Bill Dickerson said that in 2020 he began questioning claims about what AGGA could achieve and then severed all ties with Galella.
That same year, however, the Las Vegas Institute switched to Anterior Remodeling Appliance, or ARA, according to Dickerson’s Facebook posts. Dickerson has stated in several Facebook posts over the past three years that AGGA and ARA are very similar, including a June 2021 post describing ARA as “almost the same device.” He also said that most dentists associated with the Las Vegas Institute have switched to ARA, which is made in a different dental lab than AGGA.
“Another lab. Same thing,” Dickerson wrote in another Facebook post.
The Las Vegas Institute did not respond to requests for comment on the ARA. Institute attorney William Schuller had previously declined to discuss the ARA.
Dentists who have warned about AGGA have said they are also worried about ARA.
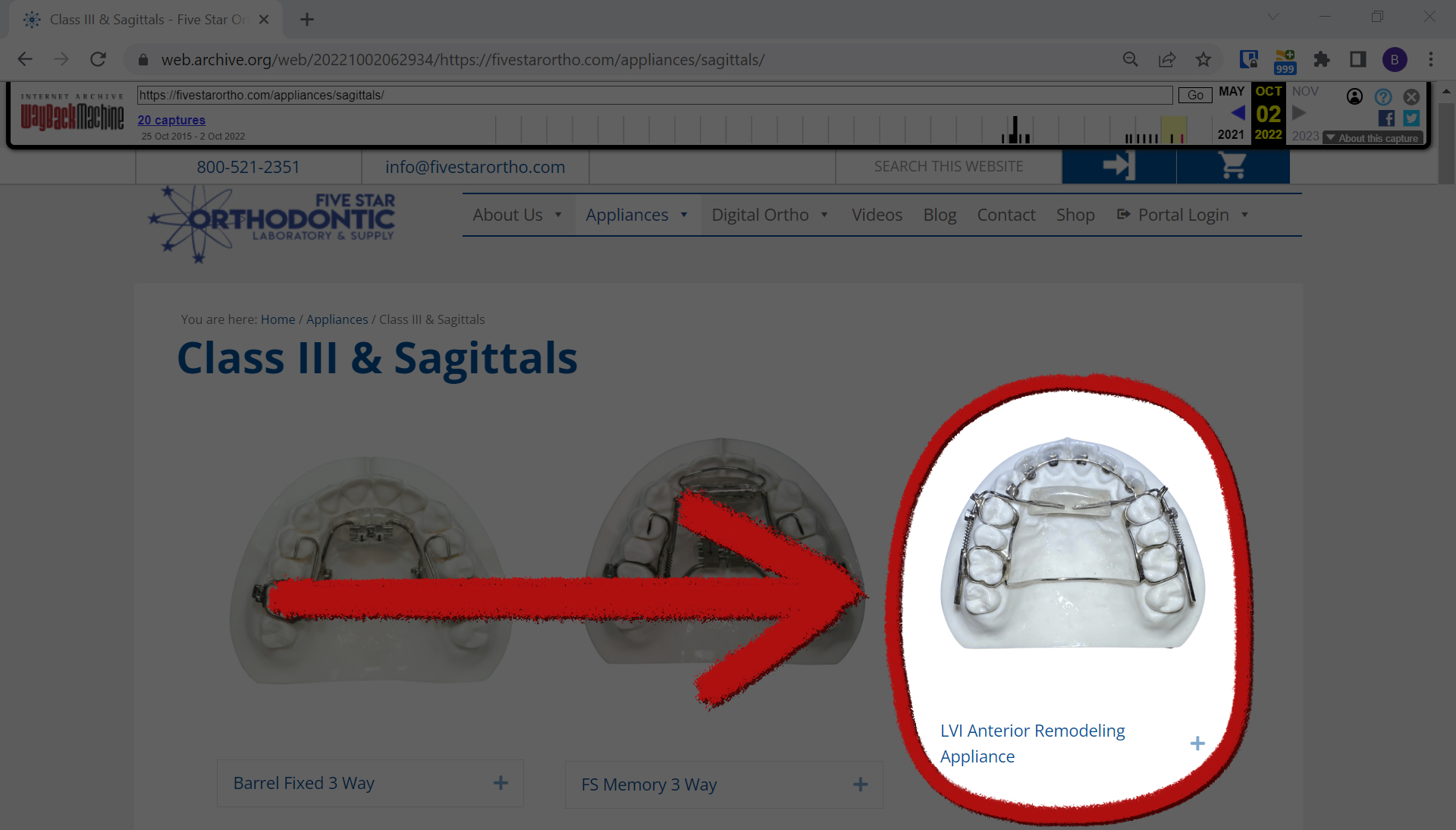
Dr. Casey Lee, a California maxillofacial surgeon, and Dr. George Mandelaris, a Chicago periodontist, each said they have examined several patients affected by AGGA, said after viewing an ARA photo from the manufacturer’s website. website, it is very similar to AGGA.
“It’s very similar to AGGA,” Mandelaris said in an email. “Almost identical.”
Dallas Press News – Latest News:
Dallas Local News || Fort Worth Local News | Texas State News || Crime and Safety News || National news || Business News || Health News









